The equilibrium 3Fe(s) + C(s) 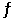 Fe3C(s) is established in a solid solution. For such a solution, one can write an equilibrium constant in the usual way except that here one has concentrations that refer to solids in the solid solution. Determine the equilibrium constant for the formation of cementite from iron and carbon at 680°C.(Given: for this reaction at 25°C, H° = 21 kJ/mol and S° = 20.4 J/mol·K)
Fe3C(s) is established in a solid solution. For such a solution, one can write an equilibrium constant in the usual way except that here one has concentrations that refer to solids in the solid solution. Determine the equilibrium constant for the formation of cementite from iron and carbon at 680°C.(Given: for this reaction at 25°C, H° = 21 kJ/mol and S° = 20.4 J/mol·K)
A) 0.75
B) 0.33
C) 3.1
D) 0.82
E) 1.2
Correct Answer:
Verified
Q25: Calcium metal is produced by electrolysis of
A)
Q26: Basic properties are characteristic of all alkaline
Q29: Which two compounds are produced by the
Q31: Which element group includes the most reactive
Q32: Write the chemical formula of calcite.
Q32: In the production of potassium metal, the
Q35: Which one of these species is an
Q36: Aluminum is an active metal, but does
Q37: Which choice gives two raw materials used
Q41: Write the chemical formula of epsomite (sold
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents