Water, 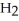 O, and methane, C
O, and methane, C 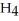 , have about the same mass and differ by only one type of atom. Why is the boiling point of water so much higher than that of methane?
, have about the same mass and differ by only one type of atom. Why is the boiling point of water so much higher than that of methane?
A) The water molecule is less symmetrical than is the methane molecule.
B) The oxygen of a water molecule has two lone pairs of electrons.
C) The electronegativity difference between oxygen and hydrogen is greater than the electronegativity difference between carbon and hydrogen.
D) all of the above
Correct Answer:
Verified
Q101: List the following bonds in order of
Q105: Which molecule is most polar?
A)S=C=S
B)O=C=O
C)O=C=S
D)These all have
Q120: Which of the following statements describes a
Q144: Which of the following molecules is the
Q145: Which of the following molecules should have
Q146: Three kids sitting equally apart around a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents