Which combination of protons, neutrons, and electrons is correct for the isotope of copper, 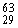 Cu
Cu
A) 29 p+, 34 n°, 29 e-
B) 29 p+, 29 n°, 63 e-
C) 63 p+, 29 n°, 63 e-
D) 34 p+, 29 n°, 34 e-
E) 34 p+, 34 n°, 29 e-
Correct Answer:
Verified
Q22: Which pair of atoms constitutes a pair
Q24: In the symbol shown below, x =
Q25: Which isotope has 36 electrons in an
Q26: An atom of the most common isotope
Q28: Gravitational forces act between objects in proportion
Q30: Which isotope has 45 neutrons?
A) 
Q31: Which atom has the largest number of
Q32: In the symbol below, X = _.
Q33: Different isotopes of a particular element contain
Q37: The subatomic particles located in the nucleus
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents