The molecular geometry of the right-most carbon in the molecule below is __________. 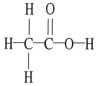
A) trigonal planar
B) trigonal bipyramidal
C) tetrahedral
D) octahedral
E) T-shaped
Correct Answer:
Verified
Q10: The basis of the VSEPR model of
Q11: The O-C-O bond angle in the CO32-
Q12: The central iodine atom in the ICl4-
Q12: The molecular geometry consists of _.
a.a nonbonding
Q14: The molecular geometry of the left-most carbon
Q16: The bond angles marked a, b, and
Q17: The central iodine atom in IF5 has
Q19: In counting the electron domains around the
Q19: The electron domain and molecular geometry of
Q20: ClF3 has "T-shaped" geometry. There are _
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents