The hybridization of the oxygen atom labeled y in the structure below is __________. The C-O-H bond angle is __________. 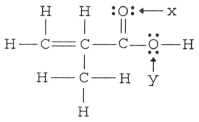
A) sp, 180°
B) sp2, 109.5°
C) sp3, 109.5°
D) sp3d2, 90°
E) sp, 90°
Correct Answer:
Verified
Q36: Of the following,the central atom is sp3d2
Q38: The hybridization scheme for BeF2 is _.
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
Q39: The hybridizations of bromine in BrF5 and
Q48: The electron-domain geometry of a carbon-centered compound
Q48: The electron-domain geometry of the AsF5 molecule
Q49: There are _ unhybridized p atomic orbitals
Q50: Of the following, only _ has sp2
Q53: Consider the following species when answering the
Q56: When three atomic orbitals are mixed to
Q59: When four atomic orbitals are mixed to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents