For the reaction HO(g) + H2(g) H2O(g) + H(g)
A plot of 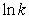 versus 1/T gives a straight line with a slope equal to -5.1 * 103 K.What is the activation energy for the reaction?
versus 1/T gives a straight line with a slope equal to -5.1 * 103 K.What is the activation energy for the reaction?
A) 42 kJ.mol - 1
B) 98 kJ.mol - 1
C) 0.61 kJ.mol - 1
D) 5.1 kJ.mol - 1
E) 12 kJ.mol - 1
Correct Answer:
Verified
Q42: A catalyst facilitates a reaction by
A) increasing
Q48: For the reaction cyclobutane(g)
Q50: Consider the dimerization reaction below: 2A
Q51: A certain reaction has a rate constant
Q52: The reaction profile for the reaction
[(CN)5CoOH2]2
Q55: What is the half-life of a second
Q56: Consider the reaction NOBr(g)
Q57: The reaction 2NO(g)+ O2(g)
Q57: Given:
CH4(g)+ Cl2(g)
Q70: The reaction 2NO2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents