The reaction 2NO(g) + O2(g) 2NO2(g) has Hr = -114 kJ.mol - 1.A possible mechanism for this reaction is 2NO(g) 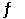 N2O2(g)
N2O2(g)
Rapid equilibrium,K
N2O2(g) + O2(g) 2NO2(g)
Slow,k
If the activation energy for the reverse reaction is 225 kJ.mol - 1,what is the activation energy for the forward reaction?
A) Because an intermediate is involved, the forward activation energy cannot be calculated with the given information.
B) 111 kJ.mol - 1
C) 339 kJ.mol - 1
D) 114 kJ.mol - 1
E) 55.5 kJ.mol - 1
Correct Answer:
Verified
Q63: Consider the reaction
A + B
Q64: Consider the following mechanism for the
Q67: Consider the reaction
2A
Q68: For a zero-order reaction,the rate constant has
Q69: The HBr synthesis is thought to
Q73: The rate law for the following
Q74: The rate law for a reaction can
Q74: For the reaction A
Q87: All of the following statements with respect
Q87: The reaction
3C + D
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents