When aqueous solutions of AgNO3 and KI are mixed,silver iodide precipitates.The balanced net ionic equation is ________.
A) Ag+ (aq) + 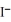 (aq) → AgI (s)
(aq) → AgI (s)
B) Ag+ (aq) + NO3- (aq) → AgNO3 (s)
C) Ag+ (aq) + NO3- (aq) → AgNO3 (aq)
D) AgNO3 (aq) + KI (aq) → AgI (s) + KNO3 (aq)
E) AgNO3 (aq) + KI (aq) → AgI (aq) + KNO3 (s)
Correct Answer:
Verified
Q64: The net ionic equation for the reaction
Q65: When H2SO4 is neutralized by NaOH in
Q66: Which ions are spectator ions in the
Q67: Combining aqueous solutions of BaI2 and Na2SO4
Q68: Which of the following are strong electrolytes?
HCl
HC2H3O2
NH3
KCl
A)HCl,
Q70: The spectator ions in the reaction between
Q71: What are the spectator ions in the
Q72: The spectator ions in the reaction between
Q73: Which one of the following substances is
Q74: What are the spectator ions in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents