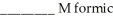 acid and
acid and 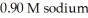 formate are required to prepare a buffer solution with a pH of 4.78 .The
formate are required to prepare a buffer solution with a pH of 4.78 .The 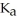 of formic acid is
of formic acid is 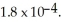
A) 0.17
B) 0.083
C) 3.3 × 103
D) 0.041
E) 9.8
Correct Answer:
Verified
Q67: 0.78 M Na Q68: What is the pH of a solution Q69: Consider a solution containing 0.100 M fluoride Q70: A buffer solution contains 0.100 M fluoride Q71: Calculate the pH of a solution prepared Q73: A 25.0 mL sample of 0.150 M Q74: What is the pH of a solution Q75: How many milliliters of 0.0839 M NaOH Q76: The addition of hydrofluoric acid and _ Q77: A solution is prepared by dissolving 0.23![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents