The standard Gibbs free energy of formation of ________ is zero.
(a) H2O (l)
(b) Fe (s)
(c) 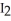 (s)
(s)
A) (a) only
B) (b) only
C) (c) only
D) (b) and (c)
E) (a) , (b) , and (c)
Correct Answer:
Verified
Q90: The normal boiling point of C2Cl3F3 is
Q91: The standard Gibbs free energy of formation
Q92: Consider the reaction: FeO (s)+ Fe (s)+
Q93: The signs of ΔH° and ΔS° must
Q94: ΔS is negative for the reaction _.
A)Sr(NO3)2
Q96: Consider the reaction between ammonia and hydrochloric
Q97: Consider the formation of solid silver chloride
Q98: Which of the following has the largest
Q99: The value of ΔG° at 181.0 °C
Q100: What is the equilibrium constant for a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents