What is the mass of 1.20 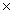 1023 molecules of CH3OH?
1023 molecules of CH3OH?
A) 161g
B) 38.5g
C) 6.39g
D) 32.1g
Correct Answer:
Verified
Q28: What is the molar mass of KClO3?
A)90.55g
B)122.55g
C)161.45g
D)6.02
Q29: What is the mass of 3.06
Q30: How many moles are present in 1.21
Q31: How many moles are present in 6.02
Q32: What is the mass of 2.00
Q34: What is the molar mass of sodium
Q35: What is the molar mass of Mg(ClO4)2?
A)223.21g
B)1.00g
C)301.01g
D)187.76g
Q36: How many molecules of CH3OH will contain
Q37: How many molecules are present in 0.300
Q38: What is the mass of 3.01
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents