What is the percent composition by mass of the substance shown below? 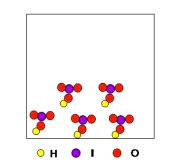
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q96: Which of the following contains the greatest
Q97: Which of the following contains the largest
Q98: A 3.000g sample of a compound is
Q99: All of the following contain the same
Q100: One mole of magnesium has the same
Q102: A sample of sodium sulfate (Na2SO4)contains 2.3
Q103: A sample of magnesium with a mass
Q104: How many carbon atoms have about the
Q105: A 205 g sample of H2Se contains
Q106: An x-gram sample of P4O6 contains 455
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents