Calculate how much ethanol,C2H5OH,will be produced from the reaction of 590.g of sugar (C6H12O6) if the reaction has a 88.5% yield. C6H12O6 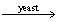 2C2H5OH + 2CO2
2C2H5OH + 2CO2
A) 302 g
B) 170.g
C) 267 g
D) 134 g
Correct Answer:
Verified
Q56: What mass of H2O is produced
Q57: How many moles of carbon monoxide
Q58: What mass of NH3 is produced
Q59: What mass of H2O is produced
Q60: What mass of CO2 will be
Q62: Calculate the percent yield for the following
Q63: In a reaction to produce chlorine gas,the
Q64: Which is the limiting reactant when
Q65: Which is the limiting reactant when
Q66: Which substances are in excess when
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents