The following table lists some compounds and their respective surface tensions at 25°C: 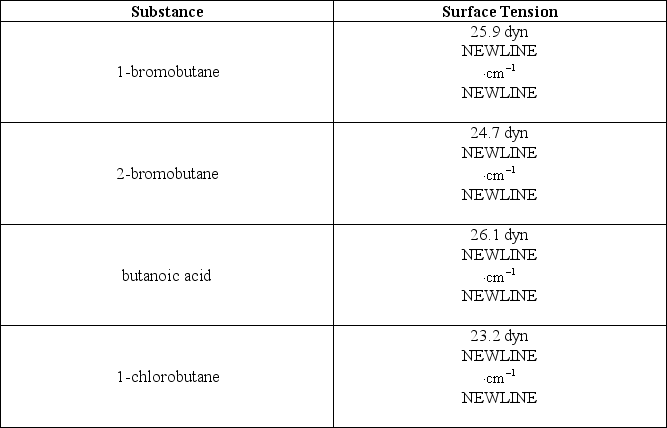 Which of the following alternatives is false?
Which of the following alternatives is false?
A) The attractive forces between molecules of butanoic acid are stronger than those between molecules of 1-chlorobutane.
B) The attractive forces between molecules of 1-chlorobutane are stronger than those between molecules of 1-bromobutane.
C) Molecules of 1-chlorobutane experience the least resistance to increase their surface area compared to any of the other compounds.
D) The compounds 1-chlorobutane,2-bromobutane,1-bromobutane,and butanoic acid are arranged in order of increasing strength of their intermolecular forces.
Correct Answer:
Verified
Q1: The following table lists normal boiling points
Q2: Which phase change corresponds to condensation?
A)Liquid to
Q3: As the volatility of a liquid increases,its
Q4: What is the vapor pressure of water
Q6: In a system at equilibrium between the
Q7: Which has the highest vapor pressure?
A)25 mL
Q8: Which phase change corresponds to sublimation?
A)Solid to
Q9: The vapor pressure of a liquid is
Q10: As the attractive forces between the molecules
Q11: Which phase change corresponds to evaporation?
A)Solid to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents