Use the following graph to select the boiling point of ethyl alcohol at 400 torr. 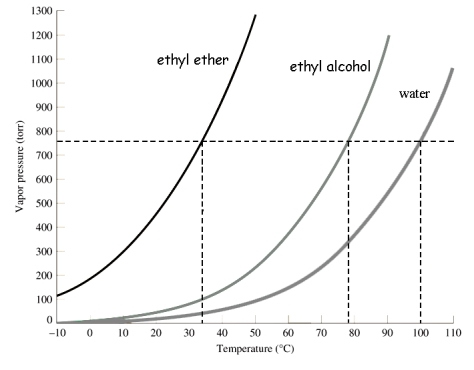
A) about 35°C
B) about 78°C
C) about 20°C
D) about 62°C
Correct Answer:
Verified
Q10: As the attractive forces between the molecules
Q11: Which phase change corresponds to evaporation?
A)Solid to
Q12: As the rate of evaporation of a
Q13: Which has the lowest vapor pressure?
A)25 mL
Q14: As the attractive forces between the molecules
Q16: What is the boiling point of water
Q17: The boiling point temperature of a liquid
Q18: As the attractive forces between the molecules
Q19: Which segment in the following figure corresponds
Q20: Which phases or states are present within
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents