Which of the following reactions would produce water?
A) Fe(s) + HNO3(aq) 
B) NaCl(aq) + KOH(aq) 
C) C3H8(g) + O2(g) 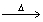
D) H2(g) + Zn(s) 
Correct Answer:
Verified
Q17: The boiling point temperature of a liquid
Q18: As the attractive forces between the molecules
Q19: Which segment in the following figure corresponds
Q20: Which phases or states are present within
Q21: Which phase change represents melting?
A)Liquid to solid
B)Solid
Q23: For any substance,which phase of matter contains
Q24: The amount of energy required to change
Q25: As the external pressure on a liquid
Q26: The freezing point of a substance is
Q27: At which external pressure will water boil
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents