What mass of ice,at 0.00 ° C,can be converted to steam,at 100. °C,if it absorbs 1.00 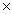 10 6 J of heat energy? The heat of fusion of ice is 335 J/g,the heat of vaporization of water is 2.26 kJ/g,and the specific heat of liquid water is 4.184 J/g v C.
10 6 J of heat energy? The heat of fusion of ice is 335 J/g,the heat of vaporization of water is 2.26 kJ/g,and the specific heat of liquid water is 4.184 J/g v C.
Correct Answer:
Verified
Q90: A 3.000 g sample of hydrated calcium
Q91: The hydrate of nitrous acid is dinitrogen
Q92: What quantity of heat energy is required
Q93: Two samples of water,one liquid and one
Q94: Evaporation is the change from the liquid
Q95: A hydrated sample of cobalt(II)chloride was analyzed
Q96: As the temperature of a liquid increases,its
Q97: At 50.° C the vapor pressure of
Q99: At pressures below one atmosphere,water will boil
Q100: When acetic acid reacts with potassium hydroxide,water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents