In order to decrease the freezing point of 500.g of water to 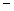 1.0°C,how many grams of ethylene glycol (C2H6O2) must be added? (Kf = 1.86
1.0°C,how many grams of ethylene glycol (C2H6O2) must be added? (Kf = 1.86 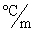 )
)
A) 5.37 g
B) 1.08 g
C) 16.7 g
D) 66.7 g
Correct Answer:
Verified
Q56: A solution is prepared by dissolving 0.400
Q57: What is the molality of a solution
Q58: A solution is prepared by mixing 20.0
Q59: What is the molarity of the resulting
Q60: What is the molarity of a solution
Q62: What is the boiling point of a
Q63: How many moles of KCl are dissolved
Q64: How would you prepare 150.0 mL of
Q65: How many grams of Zn(s)will react completely
Q66: When compared to pure water,aqueous solutions always
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents