In which direction will the point of equilibrium shift when the volume of the reaction vessel increases in the following equilibrium? 2 CO2 (g) 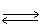 2 CO (g) + O2 (g)
2 CO (g) + O2 (g)
A) Shift to the right
B) Shift to the left
C) No shift
Correct Answer:
Verified
Q4: A solution in which equilibrium is reached
Q5: In the following equilibrium,when sodium ion is
Q6: In which direction will the point of
Q7: In which direction will the point of
Q8: In which direction will the point of
Q10: In which direction will the point of
Q11: Which is the common ion when hydrochloric
Q12: In which direction will the point of
Q13: According to Le Châtelier's Principle,decreasing the temperature
Q14: In which direction will the point of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents