Hydrocyanic acid,HCN,is a weak acid whose Ka value is 4.0 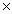 10-10.What is the pH of a 0.10 M solution of HCN?
10-10.What is the pH of a 0.10 M solution of HCN?
A) 1.0
B) 5.2
C) 9.4
D) 10.
Correct Answer:
Verified
Q45: A solution of NaNO3 would be
A)acidic.
B)basic.
C)neutral.
Q46: A solution of K2SO3 would be
A)acidic.
B)basic.
C)neutral.
Q47: Which salt would produce a neutral solution?
A)KNO2
B)NH4Cl
C)Na2S
D)KCl
Q48: What is the pH of a 0.0010
Q49: Hydrocyanic acid,HCN,is a weak acid whose Ka
Q51: Which salt would produce an acidic solution?
A)NaCl
Q52: In aqueous solution the [OH-1] is 3.0
Q53: The Ksp of silver iodide is 8.3
Q54: Hydrosulfuric acid,H2S,is a weak acid whose Ka
Q55: The pH of an aqueous solution is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents