The solubility of CaF2 is 2.14  10-4 M.The Ksp of CaF2 is
10-4 M.The Ksp of CaF2 is
A) 3.92 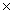 10-11
10-11
B) 4.58 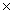 10 -8
10 -8
C) 9.80 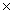 10-12
10-12
D) 2.14 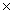 10-8
10-8
Correct Answer:
Verified
Q70: The mathematical expression for the equilibrium constant
Q71: The sodium salts of all anions listed
Q72: What is the pH of a 0.00500
Q73: The concentration of hydroxide ions, 
Q74: The solubility of silver bromide is 7.1
Q76: A 0.20 M solution of the weak
Q77: What is the pH of 0.50 M
Q78: In the following equilibrium;as the pressure is
Q79: If HCl (aq)is added to a saturated
Q80: A 0.30 M solution of formic acid,HCHO2,is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents