Which of the following is the correct Lewis structure for C2H4?
A) 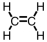
B) 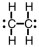
C) 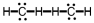
D) 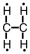
Correct Answer:
Verified
Q1: When an ionic compound forms between sodium
Q3: The ionic compound that forms between magnesium
Q7: Which of the following electronic changes will
Q8: When magnesium forms an ion,it would be
Q9: Which of the following would cause a
Q10: A correct Lewis structure for BrCl is
Q10: For some representative metals, two possible ions
Q11: If the electronegativity difference between A and
Q12: Some elements can display more than one
Q13: The correct name for CeS is _
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents