If the following generic atom were to undergo ionization, what would be the charge of the most likely product? 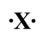
A) +2
B) -2
C) -6
D) +6
E) would probably not ionize
Correct Answer:
Verified
Q25: Why does an atom with many valence
Q37: Which of the following elements will most
Q39: If a neutral atom loses one electron,what
Q43: What is the compound that forms if
Q45: Which of the following compounds contains ionic
Q46: Which are closer together: the two nuclei
Q47: How many oxide ions (O-2)are needed to
Q47: Which of the following substances contains F-
Q49: Is an ionic compound an example of
Q49: The neon atom tends NOT to lose
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents