Given the following diagram, describe what happens electronically between these two molecules. 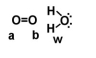
A) Oxygen A becomes slightly positively charged due to the protons on the water molecule.
B) Oxygen B becomes slightly positively charged due to the protons on the water molecule.
C) Oxygen A becomes slightly negatively charged due to the oxygen molecule.
D) Hydrogens on oxygen W becomes slightly positively charged due to the oxygen molecule.
E) none of the above
Correct Answer:
Verified
Q36: Which of the following would have the
Q122: What is a hydrogen bond?
A)a special type
Q126: Which of the following would have the
Q129: Which of the following intermolecular forces best
Q131: Which of the following intermolecular forces best
Q136: Which of the following molecules is most
Q139: Why is calcium fluoride, CaF2, a high
Q141: Chlorine, Cl2, is a gas at room
Q142: Two chemical structures are shown, one of
Q143: Plastic wrap is made of nonpolar molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents