One mole of a monatomic ideal gas at a pressure of 3.0 atm and a volume of 30 L is isothermally expanded to a pressure of 1.0 atm and a volume of 90 L.Afterwards it is compressed at a constant pressure until its volume is 30 L and then it's pressure is increased at a constant volume back to the original volume of 30 L as shown in the PV diagram.What is the efficiency of this heat engine cycle? 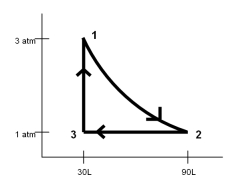
A) 0.21
B) 0.43
C) 0.57
D) 0.67
E) 0.91
Correct Answer:
Verified
Q43: You have just bought a Carnot
Q44: A 145-g snowball,initially at 0
Q45: You have just bought a Carnot
Q46: 500-g of water at 100
Q47: A 16-gram lead bullet slams into
Q49: An 800-kg car traveling at 25
Q50: What size heat pump with a coefficient
Q51: Your Carnot refrigerator has a coefficient of
Q52: Burning of fuel transfers 5 * 105
Q53: Burning of fuel transfers power into the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents