You take 50 mL of small BB's and combine them with 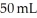 of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?
of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?
A) Since the density of the small BB's is less than that of the large BB's their volumes do not add directly to one another.
B) This is not possible since the Law of Conservation of Volume would be violated.
C) The total volume actually gets larger since mixing the BB's would leave additional air space because of the difference in size of the two BB sets.
D) Many of the smaller BB's are able to fit within the pockets of space that were empty within the 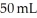 of large BB's.
of large BB's.
Correct Answer:
Verified
Q2: Aristotle described the composition and behavior of
Q5: Based on the Law of Mass Conservation,
Q6: In what sense can you truthfully say
Q6: Based on experimental evidence, John Dalton postulated
Q7: Dalton's atomic model gained credibility because _.
A)atoms
Q11: Dmitri Mendeleev's chart of elements _.
A)was used
Q13: Dmitri Mendeleev _.
A)predicted the existence of elements
Q16: A TV screen looked at from a
Q20: Red colored Kool-Aid crystals are added to
Q30: What is the mass in kilograms of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents