Germanium chloride, Ge 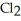 , has only two atoms surrounding the central germanium atom. Why then is the germanium chloride molecule bent?
, has only two atoms surrounding the central germanium atom. Why then is the germanium chloride molecule bent?
A) There is a covalent bond between the two chlorine atoms.
B) A lone pair of electrons on germanium pushes it to this orientation.
C) Lone pairs of electrons on the chlorine atoms push it to this orientation.
D) It is bent only periodically as it swings between both bent and linear shapes.
Correct Answer:
Verified
Q64: How many nonbonding pairs of electrons are
Q78: What does the line in the following
Q81: A hydrogen atom does not form more
Q91: Atoms of nonmetallic elements form covalent bonds,but
Q101: Which of the following molecules has a
Q104: What is the molecular shape of the
Q105: How many electrons are used to draw
Q106: How many substituents are on the following
Q107: How many electrons are used to draw
Q109: What would be the shape of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents