Would ammonia, N 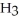 , be more or less polar if its shape were triangular planar rather than triangular?
, be more or less polar if its shape were triangular planar rather than triangular?
A) more polar because of decreased symmetry of the molecule
B) The polarity would not be affected.
C) less polar because of the increased symmetry of the molecule
D) more polar because of increased symmetry of the molecule
Correct Answer:
Verified
Q101: List the following bonds in order of
Q105: Which molecule is most polar?
A)S=C=S
B)O=C=O
C)O=C=S
D)These all have
Q120: Which of the following statements describes a
Q142: Which of the following molecules has the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents