Chlorine, 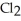 , is a gas at room temperature, but bromine,
, is a gas at room temperature, but bromine, 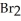 , is a liquid. Explain.
, is a liquid. Explain.
A) Chlorine atoms are larger and this makes the formation of induced dipole-induced dipole attractions more favorable.
B) Bromine atoms are larger and this makes the formation of induced dipole-induced dipole attractions more favorable.
C) The smaller chlorine molecules are able to pack together in a tighter physical orientation.
D) The bromine ions are held together by ionic bonds.
Correct Answer:
Verified
Q27: A thin stream of water is pulled
Q32: List the following compounds in order of
Q33: How are oxygen molecules attracted to water
Q35: Friends on a crowded ice skating rink
Q36: Which of the following would have the
Q38: Dipole-induced dipole forces of attraction exist between
Q121: What is the main difference between a
Q126: Which of the following would have the
Q130: Which of the following would have the
Q142: Why is the surface area of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents