Why is calcium chloride, 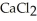 , more effective at melting ice than sodium chloride, NaCl?
, more effective at melting ice than sodium chloride, NaCl?
A) Calcium ions are both larger and more highly charged and thus more effective in breaking the ice crystal structure.
B) Calcium ions are smaller than sodium ions and thus more able to get in the way of the water molecules attempting to form the solid state lattice.
C) Calcium chloride produces three ion particles while sodium chloride produces only two. Greater numbers of ions are more effective at decreasing the number of water molecules entering the solid phase.
D) Sodium ion is a natural water softener and therefore not as effective in inhibiting the formation of the solid state of water from the liquid.
Correct Answer:
Verified
Q21: Why are polar oceans most fertile in
Q23: Consider a lake that is uniformly 10°C.
Q23: Why does adding heat to ice-water disfavor
Q24: Describe what happens to an ice cube
Q27: Which graph most appropriately shows the density
Q27: Why does ice form at the surface
Q28: Suppose that water is used in a
Q33: Why does water not freeze at 0°C
Q34: Is the density of near-freezing water, which
Q37: Why does a lake freeze from the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents