Which has the greater mass, 1.204 × 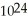 molecules of molecular hydrogen,
molecules of molecular hydrogen, 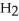 , or 1.204 ×
, or 1.204 × 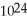 molecules of water,
molecules of water, 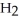 O?
O?
A) 1.204 × 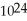 molecules of molecular hydrogen has a greater mass than 1.204 ×
molecules of molecular hydrogen has a greater mass than 1.204 × 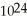 molecules of water
molecules of water
B) 1.204 × 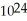 molecules of water has a greater mass than 1.204 ×
molecules of water has a greater mass than 1.204 × 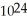 molecules of molecular hydrogen.
molecules of molecular hydrogen.
C) The mass of this many molecules of water and molecules are molecular hydrogen are the same.
D) Not enough information is provided.
Correct Answer:
Verified
Q43: What is the number of grams of
Q50: What is the number of molecules of
Q53: What is the mass of an oxygen
Q65: Which has more atoms: 64.058 g of
Q66: Which has more atoms: 17.031 g of
Q68: What is the mass of a water
Q69: What is the mass of an oxygen
Q71: What is the mass of a water
Q72: A 1.00 carat pure diamond has a
Q75: Which has the greatest number of atoms?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents