How many molecules of aspirin (formula mass aspirin = 180.0 amu) are there in a 0.250-gram sample?
A) 6.02 × 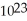
B) 8.36 × 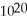
C) 1.51 × 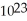
D) More information is needed.
Correct Answer:
Verified
Q36: The relative mass of carbon is 3/8
Q37: What is the formula mass of a
Q41: What is the number of moles of
Q46: What is the number of moles of
Q55: According to the following balanced chemical equation,if
Q63: Three identical candles are placed in the
Q64: Assume air has an average molar mass
Q64: Two candles of the same mass, one
Q65: Which has more atoms: 64.058 g of
Q66: Which has more atoms: 17.031 g of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents