What is the hydroxide ion concentration in an aqueous solution when the hydronium ion concentration equals 1 × 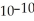 moles per liter?
moles per liter?
A) The concentration of hydroxide ions is 1 × 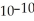 moles per liter.
moles per liter.
B) The concentration of hydroxide ions is 1 × 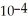 moles per liter.
moles per liter.
C) The concentration of hydroxide ions is 1 × 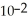 moles per liter.
moles per liter.
D) The concentration of hydroxide ions is 1 × 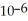 moles per liter.
moles per liter.
Correct Answer:
Verified
Q91: Lakes lying in granite basins,such as those
Q101: Carbon dioxide reacts with water to form
Q112: A buffer solution can neutralize _.
A)strong acids
Q114: Which of the following statements about buffers
Q115: When a hydronium ion concentration equals 1
Q116: Hydrogen chloride is added to a buffer
Q121: If the oceans became acidic, they would
Q128: If we were to lower our rate
Q129: Adding more carbon dioxide to the atmosphere
Q133: The overall rate at which humans are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents