In the oxidation-reduction reaction Mg(s) + 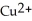 (aq) →
(aq) → 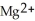 (aq) + Cu(s) , which atom or ion is reduced? Which atom or ion is oxidized?
(aq) + Cu(s) , which atom or ion is reduced? Which atom or ion is oxidized?
A) The 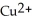 ion is oxidized as it gains electrons from the copper metal, Cu. The magnesium metal, Mg, is reduced as it loses electrons to form
ion is oxidized as it gains electrons from the copper metal, Cu. The magnesium metal, Mg, is reduced as it loses electrons to form 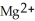 .
.
B) The 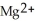 ion is reduced as it gains electrons from the copper metal, Cu. The
ion is reduced as it gains electrons from the copper metal, Cu. The 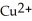 is oxidized as it loses electrons to the
is oxidized as it loses electrons to the 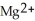 .
.
C) The 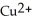 ion is reduced as it gains electrons to form copper metal, Cu. The magnesium metal, Mg, is oxidized as it loses electrons to form
ion is reduced as it gains electrons to form copper metal, Cu. The magnesium metal, Mg, is oxidized as it loses electrons to form 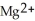 .
.
D) Since Mg goes from a solid to an aqueous solution and Cu goes from an aqueous solution to a solid, an oxidation-reduction reaction did not occur.
Correct Answer:
Verified
Q57: The zinc within a copper penny does
Q58: Upon ingestion, grain alcohol, Q59: Glucose, Q72: Zinc, Zn, is more easily oxidized than Q73: Why is the anode of a battery Q131: In a battery,the following two oxidation-reduction reactions Q134: What is the purpose of a salt-bridge? Q138: The following set of redox reactions takes Q144: What is the primary difference between a Q150: Why might disposing of a lead-acid battery,nickel-cadmium![]()
![]()
A)to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents