Light shines through atomic hydrogen gas.It is seen that the gas absorbs light readily at a wavelength of 91.63 nm.What is the value of n of the level to which the hydrogen is being excited by the absorption of light of this wavelength? Assume that the most of the atoms in the gas are in the lowest level.(h = 6.626 × 10-34 J ∙ s,c = 3.00 × 108 m/s, 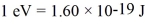 ,the Rydberg constant is R = 1.097 × 107 m-1)
,the Rydberg constant is R = 1.097 × 107 m-1)
A) 14
B) 16
C) 11
D) 21
Correct Answer:
Verified
Q3: At absolute temperature T,a black body radiates
Q4: A nonrelativistic electron and a nonrelativistic proton
Q4: If the accuracy in measuring the position
Q5: A hydrogen atom is in its n
Q6: The Bohr radius of the hydrogen atom
Q8: In the vicinity of what frequency does
Q10: The energy of the ground state in
Q11: A perfectly black body at 100°C emits
Q12: What is the frequency of the light
Q17: If the accuracy in measuring the velocity
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents