The wavelength of a ruby laser is 694.3 nm.What is the energy difference between the two energy states involved in laser action? (c = 2.9979 × 108 m/s,h = 6.626 × 10-34 J • s, 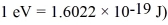
A) 1.537 eV
B) 1.646 eV
C) 1.786 eV
D) 1.812 eV
E) 3.572 eV
Correct Answer:
Verified
Q37: In a double slit experiment,a beam of
Q38: The excited state of a certain atom
Q39: Electrons emerge from an electron gun with
Q40: A nonrelativistic electron has a kinetic energy
Q41: A collection of atoms has 20% of
Q43: In a ruby laser,an electron jumps from
Q44: A 440-nm spectral line is produced by
Q45: An ultraviolet source produces a monochromatic beam
Q46: How many photons per second emerge from
Q47: A certain particle's energy is measured by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents