The process shown in the pV diagram in the figure is an 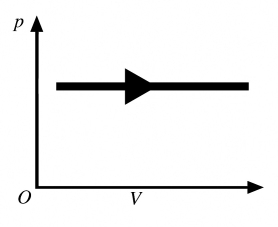
A) adiabatic expansion.
B) isothermal expansion.
C) isochoric expansion.
D) isobaric expansion.
E) isochoric compression.
Correct Answer:
Verified
Q4: When a fixed amount of ideal gas
Q5: When a gas undergoes an isothermal process,there
Q5: It is a well-known fact that water
Q9: When a vapor condenses,
A) the temperature of
Q10: The process shown in the pV diagram
Q15: A thermally isolated system is made up
Q16: When a fixed amount of ideal gas
Q17: Heat is added to a pure substance
Q30: A quantity of ideal gas requires 800
Q40: An adiabatic compression is performed on an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents