The concentration-time dependence is shown below for two first-order reactions is: 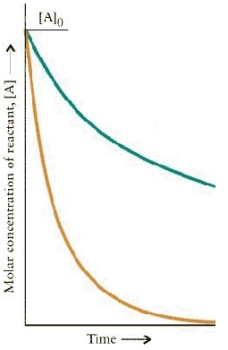
Which reaction has the larger rate constant?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q10: It is important to distinguish between the
Q15: The reaction 2NO(g)+ 2H2(g)
Q16: Given: 2A(g)+ B(g)
Q19: For the reaction 2A + B
Q20: If the rate of reaction increases by
Q21: Consider the reaction 2N2O5(g)
Q22: The concentration-time curves for two sets of
Q23: For the reaction A
Q24: For the reaction cyclopropane
Q25: For a given first-order reaction,after 2.00 min,20%
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents