Which one of the statements below is concerning the following reaction:
NH3(g) + H2O 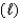 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
A) The double arrows indicate that ammonia,NH3,is only very slightly soluble in water.
B) The reaction is reversible.
C) When NH3 is added to H2O,NH4+ and OH- ions are produced in a 1:1 ratio.
D) When solutions of NH4+ and OH- are mixed,some ammonia is produced.
E) Ammonia partially reacts with water.
Correct Answer:
Verified
Q5: Which of the following statements is/are CORRECT?
1)Most
Q6: The complete combustion of nonane,C9H20,yields carbon
Q7: A precipitate is expected when an aqueous
Q8: Which of the following compounds is
Q9: Nitroglycerin decomposes violently according to the
Q11: Metals react with oxygen gas to
Q12: Which of the following statements is/are CORRECT?
1)Water
Q13: Which of the following statements concerning electrolytes
Q14: What is the balanced chemical equation
Q15: Which of the following statements is/are CORRECT?
1)A
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents