If 0.1800 g of impure soda ash (Na2CO3) is titrated with 15.66 mL of 0.1082 M HCl,what is the percent purity of the soda ash?
Na2CO3(aq) + 2 HCl(aq) 2 NaCl(aq) + H2O( 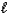 ) + CO2(g)
) + CO2(g)
A) 17.96%
B) 49.89%
C) 50.11%
D) 94.13%
E) 99.77%
Correct Answer:
Verified
Q60: What mass of Na2CO3 is present in
Q62: A 50.00-mL sample of a weak
Q62: The reaction of 5.07 g N2 with
Q63: A solution of sodium oxalate (Na2C2O4)in
Q66: The percent yield of a chemical reaction
Q66: In combustion analysis,the gases produced by the
Q67: An unknown diprotic acid (H2A)requires 44.39 mL
Q67: The pH of purified water (or of
Q69: An impure sample of benzoic acid
Q71: The numbers preceding the formulas in chemical
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents