To solve problems using Charles's Law,which mathematical equation should be used?
A) 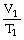
=
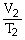
B) T1V1 = T2V2
C) 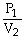
=
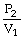
D) P2V1 = P1V2
E) none of the above
Correct Answer:
Verified
Q21: If the number of gas particles is
Q22: If you had a five liter balloon
Q24: The vapor pressure of water is independent
Q36: The main component of the air we
Q43: The initial volume of a gas cylinder
Q44: To solve problems using Boyle's Law,which mathematical
Q45: Divers often inflate heavy duty balloons attached
Q50: What is the equivalent pressure of 760
Q56: 1 torr is equal to:
A)760 mm Hg.
B)1
Q59: 1 atm is equal to:
A)760 mm Hg.
B)760
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents