The structure 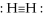
is a proper representation of the bonding in the H2 molecule.
Correct Answer:
Verified
Q1: When calculating the number of electrons for
Q2: Li : is the proper Lewis structure
Q7: A correct Lewis structure for an atom
Q9: The Lewis structure for O2 contains a
Q12: The triple bond present in diatomic nitrogen,N2,is
Q15: Having eight valence electrons is very stable
Q16: The Lewis theory predicts that the formula
Q18: Drugs to fight HIV have been developed
Q26: The VSEPR theory predicts that the H-C-H
Q39: The correct Lewis structure for CO2 shows
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents