How many joules of heat are needed to completely vaporize 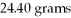
Of water at its boiling point?
Given 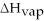
= 40.6 kJ/mol
A) 54.97
B) 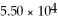
C) 29.98
D) 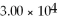
E) none of the above
Correct Answer:
Verified
Q62: The ability of sodium chloride to mix
Q64: The amount of heat required to melt
Q67: Which substance below has dipole-dipole forces?
A)CH4
B)CO2
C)F2
D)H2S
E)none of
Q68: Liquids that have high vapor pressure and
Q69: Which statement is TRUE in describing what
Q70: When sufficient quantity of heat has been
Q73: How many kilojoules of heat are needed
Q73: In northern climates,it is common to have
Q77: Which intermolecular force is present in all
Q78: What happens as you start to add
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents