Dry ice (solid C 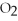
) is which type of solid?
A) molecular solid
B) ionic solid
C) covalent atomic solid
D) nonbonding atomic solid
E) metallic atomic solid
Correct Answer:
Verified
Q64: The amount of heat required to melt
Q67: Which substance below has dipole-dipole forces?
A)CH4
B)CO2
C)F2
D)H2S
E)none of
Q68: Liquids that have high vapor pressure and
Q77: Which intermolecular force is present in all
Q81: Which intermolecular force found in 
Q83: Which molecule below has hydrogen bonding?
A)N
Q84: Which compound will have the highest boiling
Q85: Which noble gas has the highest boiling
Q89: Which substance would be expected to have
Q95: Assuming that the molecules carbon monoxide (CO)and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents