How many kJ of heat are needed to completely melt 95.3 g of copper metal,given that the metal is at its melting point? The heat of fusion for this metal is 13.1 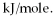
A) 19.6
B) 43.0
C) 1250
D) 0.114
E) none of the above
Correct Answer:
Verified
Q93: Which intermolecular force is common to all
Q96: Substance A is a molecular compound that
Q98: Which intermolecular forces are found in
Q99: Which compound in liquid form will have
Q103: A 250 gram sample of water at
Q103: What are the principal forces holding ice
Q107: How many kJ of heat are needed
Q108: Silicon is which type of solid?
A)molecular solid
B)ionic
Q109: Copper is which type of solid?
A)molecular solid
B)ionic
Q114: Which atomic solid has the highest melting
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents