What is the concentration of the hydroxide ion given that the concentration of the hydronium ion is 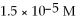
?
A) 6.7 × 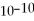
M
B) 1.5 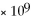
M
C) 1.0 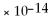
M
D) 1.0 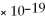
M
E) none of the above
Correct Answer:
Verified
Q66: A neutralization reaction between KOH (aq)and H2SO4
Q68: Which of the following statements about water
Q73: A substance that acts as an acid
Q74: A 35.0 mL sample of 0.225 M
Q76: Which of the following is a weak
Q82: What is the concentration of hydronium ions
Q86: What is the concentration of H⁺ in
Q94: What is the concentration of H⁺ in
Q96: In order for a solution to be
Q99: Consider a 1.6 × 10-3 M solution
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents