A large equilibrium constant 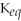
indicates that the forward reaction is largely favored.
Correct Answer:
Verified
Q1: Collisions between reactant molecules do not always
Q2: Placing a [ ] around the formula
Q3: Equilibrium involves the ideas of sameness and
Q4: Living things are in equilibrium with their
Q7: Dynamic equilibrium occurs when the rate of
Q12: It is not necessary to have a
Q17: A reversible reaction is one that can
Q22: Decreasing the amount of carbon dioxide in
Q24: Adding heat to an exothermic reaction will
Q30: Le Chatelier's principle states that when a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents