If 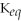
equals about ≈1,then neither direction is favored and significant amounts of both reactants and products are present at equilibrium.
Correct Answer:
Verified
Q22: Decreasing the amount of carbon dioxide in
Q23: If you have a chamber of gases
Q24: A reaction that has Q24: Adding heat to an exothermic reaction will Q25: In an exothermic reaction,you can consider the Q28: Decreasing the volume of the system below Q29: The equilibrium expression for the reaction: Q30: Le Chatelier's principle states that when a Q31: Increasing the amount of carbon in the Q35: For a given chemical equation,the coefficients for![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents