Calculate the equilibrium constant for the reaction below given that 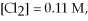
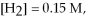
And 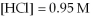
At equilibrium.
Given: 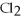
(g) + 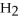
(g) ⇌ 2HCl(g)
A) 0.017
B) 0.018
C) 58
D) 55
E) none of the above
Correct Answer:
Verified
Q104: Comparing a reaction with a catalyst to
Q112: What is the [ Q112: Which statement about activation energy is FALSE? Q113: What is the concentration of the Q114: What is the value for the Q114: Which of the following is FALSE about Q115: What is the value for the Q117: What is the Q118: What happens to the equilibrium constant when Q118: What is the [ Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
A)Activation![]()
![]()

