Identify the oxidizing agent in the following reaction: 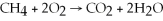
.
A) C 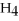
B) 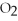
C) C 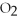
D) 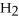
O
E) none of the above
Correct Answer:
Verified
Q23: Corrosion can be defined as the reduction
Q25: The driving force that causes electrons to
Q27: Alkaline batteries are used in automobiles.
Q28: The cathode is the electrode at which
Q37: To achieve the largest battery voltage possible,the
Q42: For the reaction Si (s)+ O2 (g)→
Q45: Oxidation typically involves:
A)the loss of electrons.
B)the loss
Q49: Rusting of iron requires the presence of
Q54: The reducing agent typically:
A)gains electrons.
B)always remains unchanged
Q59: Reduction involves which of the following?
1.Loss
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents